What are some organic chemicals. Halogen ˈhæləˌdʒɛn noun any of the chemical elements fluorine chlorine bromine iodine and astatine. What is the meaning of halogen compound.
What Is The Meaning Of Halogen Compound, These are binary compounds formed when halogens react with hydrogen. Organic chemicals are chemical compounds that contain carbon as part of their molecular structure. Halogen ˈhæləˌdʒɛn noun any of the chemical elements fluorine chlorine bromine iodine and astatine. The halogen elements are fluorine F chlorine Cl bromine Br iodine I astatine At and tennessine Ts.
 Chem4kids Com Elements Periodic Table Halogens From chem4kids.com
Chem4kids Com Elements Periodic Table Halogens From chem4kids.com
They are colourless relatively odourless and hydrophobic. In general the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. A halogen is one of a group of chemical elements that includes chlorine fluorine and iodine. Other simple organohalogens include bromomethane CH 3 Br chloroform CHCl 3.
Definition of halogen.
Read another article:
From the periodic table the halogens are the group 7 elements. Organic halogen compounds are a large class of natural and synthetic chemicals that contain one or more halogens fluorine chlorine bromine or iodine combined with carbon and other elements. Halogenated compounds or organic halides are organic compounds that contain halogen atoms. Due to this nature they can form different compounds such as halides interhalogens and polyhalogenated compounds. A halogen is one of a group of chemical elements that includes chlorine fluorine and iodine.
 Source: pinterest.com
Source: pinterest.com
We will look at them in brief below. Organic halogen compounds are a large class of natural and synthetic chemicals that contain one or more halogens fluorine chlorine bromine or iodine combined with carbon and other elements. The word halogen comes from the Greek roots hal- meaning salt and -gen meaning to produce. Halogen any of the six nonmetallic elements that constitute Group 17 Group VIIa of the periodic table. Grignard Reaction Chemical Reactions Reactions Chemical.
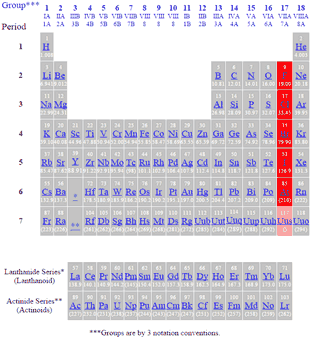 Source: gordonengland.co.uk
Source: gordonengland.co.uk
Addition of halogens to an Organic compound. In general the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. The halogen atoms react to form an interhalogen compound. In the periodic table Halogens occupy the seventh column which contains fluorine chlorine bromine iodine and astatine. Periodic Table Of The Elements Halogens.
 Source: scienceclarified.com
Source: scienceclarified.com
Low smoke zero halogen or low smoke free of halogen LSZH or LSOH or LS0H or LSFH or OHLS or ZHFR is a material classification typically used for cable jacketing in the wire and cable industry. Due to this nature they can form different compounds such as halides interhalogens and polyhalogenated compounds. The Hydrogen Halides HX The hydrogen halides are compounds that contain hydrogen attached to one of the halogens HF HCl HBr and HI. Halogenation is a reaction that occurs with the addition of one or more halogens to a substance. Halogens Humans Body Used Water Process Earth Life Plants.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The Hydrogen Halides HX The hydrogen halides are compounds that contain hydrogen attached to one of the halogens HF HCl HBr and HI. The chemistry of the halogens is dominated by oxidation-reduction reactions. Definition of halogen. The word halogen comes from the Greek roots hal- meaning salt and -gen meaning to produce. Halogens Chemistry For Non Majors.
 Source: chem4kids.com
Source: chem4kids.com
The chemistry of the halogens is dominated by oxidation-reduction reactions. A name is given considering the connectivity of the carbon atom to which the halogen is attached. In regards to Halogen Content we are specifically talking about Chlorine Fluorine Bromine Iodine and Astatine elements which have been commonly used in the production of electronics and plastics such as PVC and Teflon. From the periodic table the halogens are the group 7 elements. Chem4kids Com Elements Periodic Table Halogens.
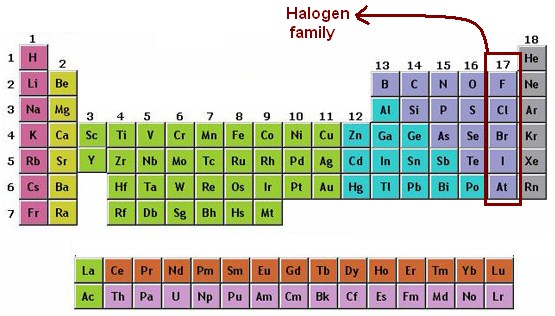 Source: alevelchemistry.co.uk
Source: alevelchemistry.co.uk
The suffix of the name reflects the type s of functional group s. The simplest organochlorine compound is chloromethane also called methyl chloride CH 3 Cl. The halogen atoms react to form an interhalogen compound. In the human body some halogens perform multiple regulatory functions while others are not essential. Halogens Facts Definition A Level Chemistry Revision Notes.
 Source: pinterest.com
Source: pinterest.com
When a hydrogen atom is directly attached to an aromatic ring is replaced by a halogen atom the compound obtained is called nuclear halogen derivative of arene or. Halogen any of the six nonmetallic elements that constitute Group 17 Group VIIa of the periodic table. In general the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. These compounds are all. Halogenation Easy Science Hydrogen Atom Functional Group Easy Science.
 Source: pinterest.com
Source: pinterest.com
These names are listed within the discussion of naming alkanes. So when something is halogenated it means the compound contains a halogen Ioding Chlorine Bromine Fluorine devi f. They are colourless relatively odourless and hydrophobic. On a global scale natural sources the sea in particular give rise to most of the chlorine bromine and iodine compounds in the atmosphere. Chlorine Chemistry Lessons Chemical Elements Periodic Table Fun Science.
 Source: britannica.com
Source: britannica.com
These names are listed within the discussion of naming alkanes. LSZH cable jacketing is composed of thermoplastic or thermoset compounds that emit limited smoke and no halogen when exposed to high sources of heat. In other cases a halogen atom acts with another lower interhalogen to form an interhalogen compound. From the periodic table the halogens are the group 7 elements. Halogen Elements Examples Properties Uses Facts Britannica.
 Source: elevise.co.uk
Source: elevise.co.uk
The halogenoalkanes or alkyl halides are a group of organic compounds derived from alkanes containing one or more halogens. One example includes the reaction when a volume of chlorine reacts with an equal volume of fluorine at 473K. Halogens Halogens are a family of chemical elements that includes fluorine F chlorine Cl bromine Br and iodine I. Due to this nature they can form different compounds such as halides interhalogens and polyhalogenated compounds. C1 N Group 7 Elements Aqa Combined Science Trilogy Elevise.
 Source: chemistryexplained.com
Source: chemistryexplained.com
A name is given considering the connectivity of the carbon atom to which the halogen is attached. Organohalogens are synthesized through the nucleophilic abstraction reaction. A name is given considering the connectivity of the carbon atom to which the halogen is attached. We will look at them in brief below. Organic Halogen Compounds Chemistry Encyclopedia Reaction Elements Examples Gas Number Atom Synthesis Reactivity.
 Source: britannica.com
Source: britannica.com
A halogen is one of a group of chemical elements that includes chlorine fluorine and iodine. These are binary compounds formed when halogens react with hydrogen. Organohalogens are synthesized through the nucleophilic abstraction reaction. A name is given considering the connectivity of the carbon atom to which the halogen is attached. Halogen Elements Examples Properties Uses Facts Britannica.
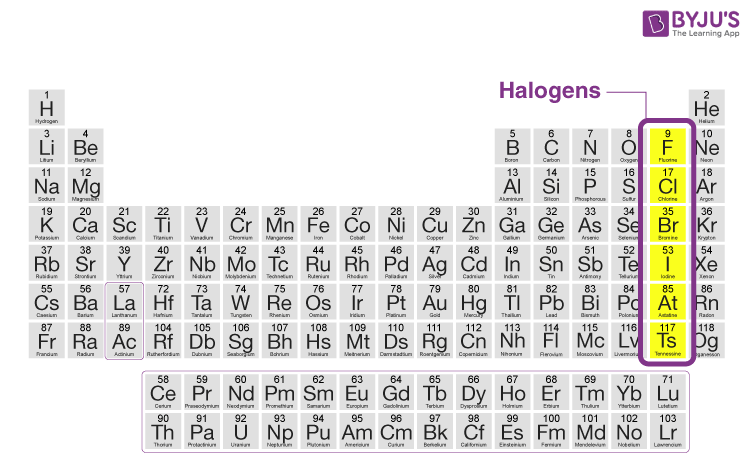 Source: byjus.com
Source: byjus.com
These are binary compounds formed when halogens react with hydrogen. Organohalogens are synthesized through the nucleophilic abstraction reaction. The interesting thing about these compounds is the carbon-halogen bond and all the nucleophilic substitution reactions of the halogenoalkanes involve breaking that bond. One of the definitive properties of Halogens is that they are highly reactive. Halogens Definition Uses Compounds Properties Of Halogens.
 Source: pinterest.com
Source: pinterest.com
Halogens Halogens are a family of chemical elements that includes fluorine F chlorine Cl bromine Br and iodine I. Organohalogens are synthesized through the nucleophilic abstraction reaction. The chemistry of the halogens is dominated by oxidation-reduction reactions. When a hydrogen atom is directly attached to an aromatic ring is replaced by a halogen atom the compound obtained is called nuclear halogen derivative of arene or. Alkyl Halides And Elimination Reactions Organic Reactions Reactions Functional Group.
 Source: britannica.com
Source: britannica.com
Halogens Halogens are a family of chemical elements that includes fluorine F chlorine Cl bromine Br and iodine I. LSZH cable jacketing is composed of thermoplastic or thermoset compounds that emit limited smoke and no halogen when exposed to high sources of heat. Definition of halogen. In regards to Halogen Content we are specifically talking about Chlorine Fluorine Bromine Iodine and Astatine elements which have been commonly used in the production of electronics and plastics such as PVC and Teflon. Halogen Elements Examples Properties Uses Facts Britannica.









